Negative Specific Heat: The Danes Melt a Copper Cluster. (65)
While in Denmark on sabbatical, my hosts ( Karsten Jacobsen, Jens Nørskov and their group) were simulating a 17,000 atom copper cluster using effective medium theory on a massively parallel CM-2 computer. Starting from a crystal at low temperatures, they were adding heat and watching the temperature as the cluster melted.
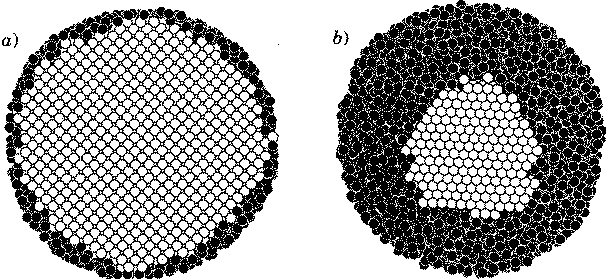
For an infinite system, the temperature should stay fixed while the crystalline core of the cluster slowly melts. Because we had a finite droplet, this jump in energy at fixed temperature becomes a curve, which in a small region bends backward: a negative specific heat! As energy is added, the crystalline nucleus melts; the smaller nucleus has a larger curvature, lowering its melting temperature. Putting in data for real copper into this simple theory led to a beautiful correspondence with the simulation.
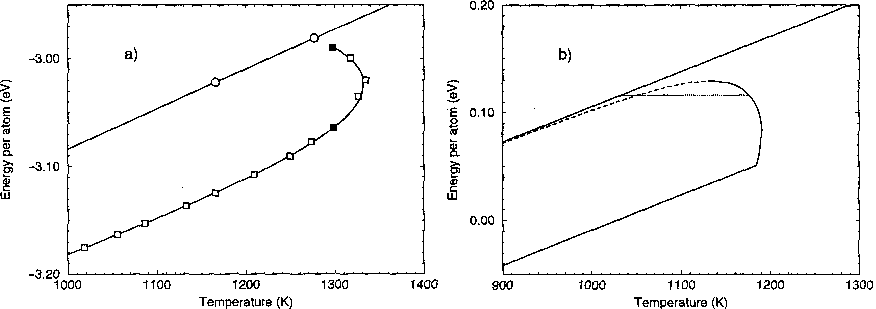
From a formal statistical mechanics viewpoint, this temperature drop scales as N^{-1/4}, where N is the number of atoms in the droplet; for large N ours is the largest finite--size effect known in first--order transitions. A macroscopic cluster with a mole of copper should have over a millidegree drop in temperature as heat is added.

