What are smectic liquid crystals?
Smectic liquid crystals are one of the spectacular surprises in materials physics. Pour this fluid into a glass container, and it forms beautiful ellipses and hyperbolas (see figure at right). The smectic fluid has a local ordering into layers, and the ellipses you see are defects -- places where the desired ordering breaks down.
Liquid crystals can be poured like liquids, but share some of the regular ordering of solid crystals. Smectic liquid crystals have layers of molecules. Molecules or atoms in a crystal are arranged into a regular grid; a cube of salt has rows, columns, and stacks of atoms along x, y, and z. Smectic liquid crystals have molecules that form layers only along one direction.
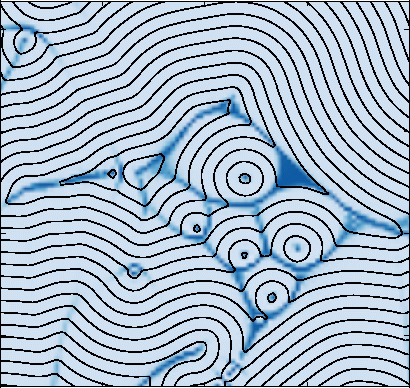
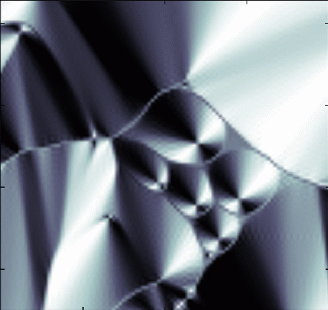 These layers don't always lie straight and flat: as the smectic grows and attaches to surfaces, the layers often bend (computer simulated figure far left). We call the regions where the layers bend abruptly defects, since they represent regions where the local layered ordering breaks down. The figure near and to the left shows how these layers would be viewed through a microscope. Notice that the defects in the center form approximate ellipses, with another defect at the focus of the ellipse.
These layers don't always lie straight and flat: as the smectic grows and attaches to surfaces, the layers often bend (computer simulated figure far left). We call the regions where the layers bend abruptly defects, since they represent regions where the local layered ordering breaks down. The figure near and to the left shows how these layers would be viewed through a microscope. Notice that the defects in the center form approximate ellipses, with another defect at the focus of the ellipse.
