Capillary action
Assuming the radius of a capillary is small and the fluid inside forms
a hemispherical meniscus at the surface - further motion through the capillary
is resisted because of a pressure gradient across the interface .
delta P is ~ Y/R :(Laplace's Formula)-
smaller the radius of the pore
(capillary) greater the resisting pressure .
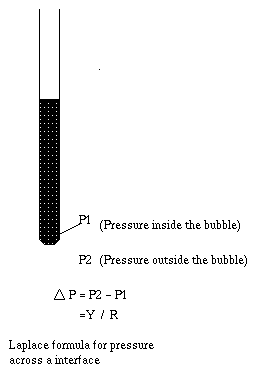
Thus the force (= pressure gradient) preventing further motion of the drop can be decreased
by
- decreasing the interfacial tension ( Y )
- increasing the radius of the capillary pore (R).
Pressure gradient
Change of pressure across unit length.
Think of pressure gradient as a force.
in vivo
(relating to a biological process). Occurring or made to occur within a
living organism or natural setting.
Opposite to in vitro.
in vitro
(relating to a biological process). Made to occur in
a laboratory vessel or other controlled experimental environment.
Opposite to in vivo.

