 |
Micelles
How do micelles act as detergents?
In the micelle phase of a mixture of surfactant and water,
if impurities like oil or soil/dirt are present the oliphilic (i.e oil
loving
- the same as water hating or
hydrophobic ) portion of the micelle surrounds the oil drop
or loosens the dirt from the underlying surface to be cleaned.
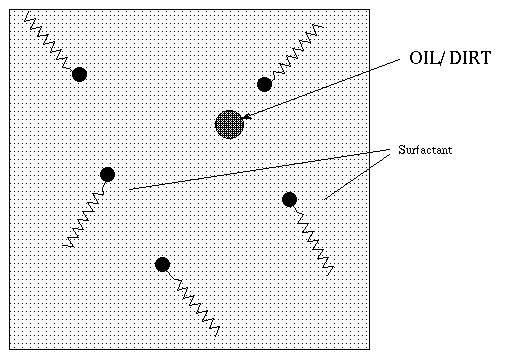
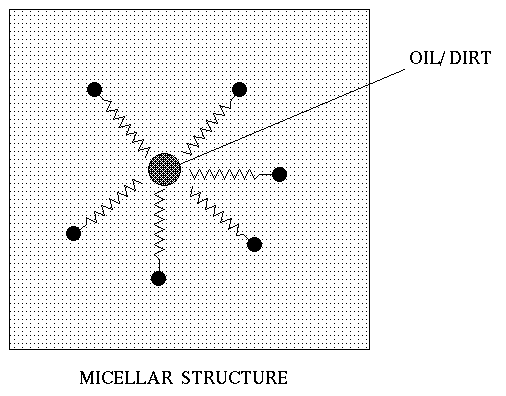 The micelles do not let a separate
oil-rich phase form hence get washed away with the water and foam .
(Note: The foam that we associate with detergents is actually due the presence
of amphiphilic molecules.)
The micelles do not let a separate
oil-rich phase form hence get washed away with the water and foam .
(Note: The foam that we associate with detergents is actually due the presence
of amphiphilic molecules.)
Generally the detergent contains about 200- 600 p.p.m
of
surfactant - but this can vary significantly with temperature, the nature of
surfactant used and the nature of the impurities. Hence different
surfactants have varying efficiencies for different situations - thus some
thought should go into deciding the optimal surfactant (detergent) to be
used for a given purpose.
|




