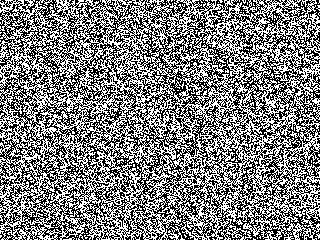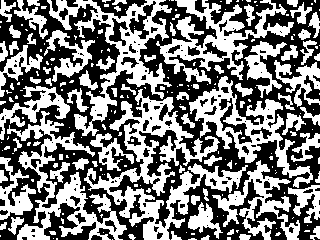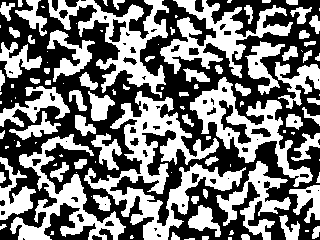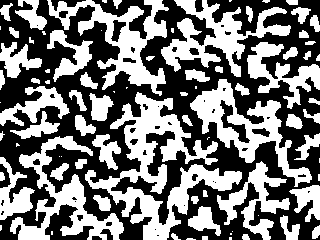 Starting from a random configuration, the black and white regions
separate from one another. The animation at left
shows the steps one at a time.
Starting from a random configuration, the black and white regions
separate from one another. The animation at left
shows the steps one at a time.
When you shake up the salad dressing, the oil and vinegar get jumbled together in small droplets. When you stop shaking, the tiny droplets merge into bigger ones, gradually making for a coarser and coarser mixture until all the oil is on the top.
Molten iron, before it is cast, has a fair percentage of carbon dissolved in it. As it cools, this dissolved carbon precipitates out (just like water droplets condense out of damp air as it cools, forming clouds and rain and snow and other precipitation). Because it cools over a period of seconds to hours, the carbon doesn't have a chance to float to the top: it stays dispersed through the iron in small particles. The hardness and brittleness of cast iron depends on the size and form of these carbon particles, and thus depends on how the iron is cooled.
Rocks often have lots of tiny grains of different materials: quartz, alkali feldspar, and plagioclase in granite; plagioclase feldspar and calcium-rich pyroxene in basalt, ... Different rocks have different sizes of these grains. Rocks formed from lava of erupting volcanos have very fine grains: molten rock cooling over eons deep underground form large grains.
Much recent progress has been made by studying simple models. The most thoroughly studied system is shown in the following pictures. Think of the black regions as oil and the white regions as vinegar: the model changes pixels from black to white if they have more vinegar neighboring pixels than oily neighbors, and vice-versa.
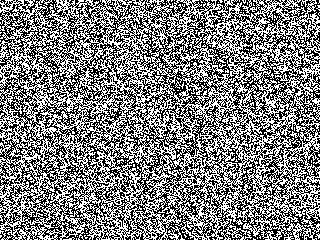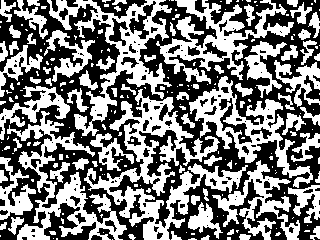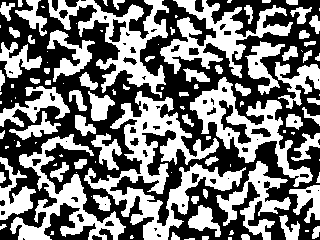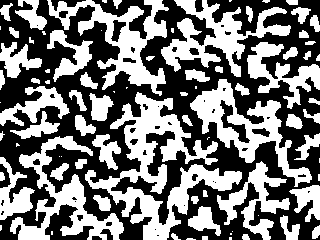 Starting from a random configuration, the black and white regions
separate from one another. The animation at left
shows the steps one at a time.
Starting from a random configuration, the black and white regions
separate from one another. The animation at left
shows the steps one at a time.
 This picture at right shows the system after two hundred more time steps.
Notice that it looks roughly like the first picture, except magnified
by a factor of about three. The animation shows the evolution from
the first picture to the second one, but speeded up to ten steps at a time.
This picture at right shows the system after two hundred more time steps.
Notice that it looks roughly like the first picture, except magnified
by a factor of about three. The animation shows the evolution from
the first picture to the second one, but speeded up to ten steps at a time.
 This picture looks like the first picture blown up by a factor of ten.
(Or, it would if we could do a 3200x2400 size simulation!) It's coarser
by ten because it's had more time for the black and white regions to
separate. It's had 100x the amount of time to separate: the length scale
grows as the square root of time. The animation shows the evolution
speeded up to 100 steps per snapshot.
(Actually, it's had 111 times as much time to separate: sorry, I should
have had 18 frames in the last two movies... Also, the periodic boundary
conditions may have caused the coarsening to accelerate at the end.)
This picture looks like the first picture blown up by a factor of ten.
(Or, it would if we could do a 3200x2400 size simulation!) It's coarser
by ten because it's had more time for the black and white regions to
separate. It's had 100x the amount of time to separate: the length scale
grows as the square root of time. The animation shows the evolution
speeded up to 100 steps per snapshot.
(Actually, it's had 111 times as much time to separate: sorry, I should
have had 18 frames in the last two movies... Also, the periodic boundary
conditions may have caused the coarsening to accelerate at the end.)
In this model, to reach a given length we need to wait a time that goes like the length times itself. (After 10x10 time steps, the length grew by a factor of 10; to get to a length 30 would take 900 time steps.) This can be explained (to a physicist, anyhow) using the fact that the boundaries between the two phases become smoothly curved at late times. Regions which curve inward tend to grow; regions which poke out shrink; small droplets shrink because they're surrounded by boundaries with mostly positive curvature. The growth gets slower as the length increases because the curvatures of the remaining black and white (oil and vinegar) regions becomes smaller and smaller. In a more realistic model (which doesn't let oil transform into vinegar, but just lets it move around) to get to a length 10 takes a time of 10x10x10: to get to 30 would take 27,000 time steps. In this model (where the number of each particle is conserved) coarsening is often associated with spinodal decomposition.
You might think that this is only the beginning: after all, black and white squares are far removed from molecules of oil and vinegar, not to mention quartz and orthoclase. Actually, in many ways this absurdly simple model is almost good enough: it is believed that the patterns of black and white in our model (at least in a more realistic version) begin to look just the same as those found in oil and water, after the domains have enough time to grow big! (Rocks are now thought to be more complicated.) The fact that things are simpler when zillions of atoms all do their own things is one of the basic truths that make science possible.
If the simplest model is so successful, what more is there to do? Actually, this is still a rather lively field: just within our group at LASSP we've had several exciting developments:
![]() Jim Sethna, sethna@lassp.cornell.edu
Jim Sethna, sethna@lassp.cornell.edu
![]() Statistical Mechanics: Entropy, Order Parameters, and Complexity,
now available at
Oxford University Press
(USA,
Europe).
Statistical Mechanics: Entropy, Order Parameters, and Complexity,
now available at
Oxford University Press
(USA,
Europe).
coarsening page