 This speedup with temperature is often found to have an activated,
or Arrhenius form:
This speedup with temperature is often found to have an activated,
or Arrhenius form:
 This speedup with temperature is often found to have an activated,
or Arrhenius form:
This speedup with temperature is often found to have an activated,
or Arrhenius form:
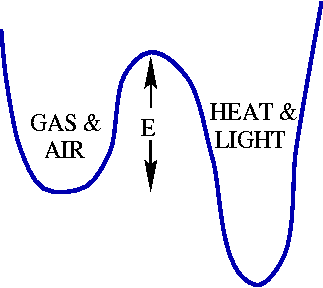
What is the physical (or chemical) explanation for this formula? Well, the gasoline and oxygen are happy, tightly bound molecules just the way they are. The atoms would be even happier if they could figure out to rearrange into carbon dioxide and water: this difference in happiness is the energy given off when the gasoline burns. In the picture, we see that the gasoline and air mixture we show in a little bowl or well, where the energy is fairly low. The other well represents the burned-up gasoline, and is even lower: the difference in height is the energy released. The main thing to notice is that there is a mound, or barrier between the two wells! The gasoline and oxygen have to get torn apart and mushed together in unhappy ways before they can reach their best state. The barrier represents the easiest way to get from one state to another. The height of this barrier is E in Arrhenius' formula.
The idea is that at high temperatures, the atoms wiggle fast, and the gasoline and oxygen can occasionally wiggle over the barrier. At low temperatures, they can only vibrate a bit inside their wells, and the reaction doesn't go. The particular formula Speed = C exp(-E/T) comes from the amazing physics truth that at temperature T, every possible configuration of atoms with energy E occurs with probability proportunate to exp(-E/T).
James P. Sethna, sethna@lassp.cornell.edu
![]() Statistical Mechanics: Entropy, Order Parameters, and Complexity,
now available at
Oxford University Press
(USA,
Europe).
Statistical Mechanics: Entropy, Order Parameters, and Complexity,
now available at
Oxford University Press
(USA,
Europe).