 Joachim Jacobsen,
Karsten Jacobsen,
and I
worked out the rate of double-jumps on surfaces.
Joachim Jacobsen,
Karsten Jacobsen,
and I
worked out the rate of double-jumps on surfaces.
An atom sitting on top of a surface will diffuse around. Usually,
the atom moves from one site to the next, squeezing its way between
two atoms in the way. We call this a single-jump. The rates
for single jumps are understood to have an
Arrhenius form
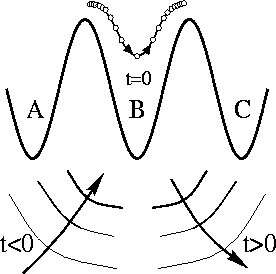 Sometimes, an atom on a surface will jump two sites at a time. That is,
the atom would jump from well A through well B, and land in well C in the
figure on the left. The height of the barriers between the three wells is
just E1: to make a single jump, the atom has to
be wiggled up to the top of the barrier, and statistical mechanics says the
probability of finding an atom at the top is proportional to
exp(-E1/kT). The Aarhus
experiment measured what fraction of the time this happened, and found that
it seemed to obeyed an equation a lot like the one for
r1 above, but with a higher energy barrier
E2!
Sometimes, an atom on a surface will jump two sites at a time. That is,
the atom would jump from well A through well B, and land in well C in the
figure on the left. The height of the barriers between the three wells is
just E1: to make a single jump, the atom has to
be wiggled up to the top of the barrier, and statistical mechanics says the
probability of finding an atom at the top is proportional to
exp(-E1/kT). The Aarhus
experiment measured what fraction of the time this happened, and found that
it seemed to obeyed an equation a lot like the one for
r1 above, but with a higher energy barrier
E2!
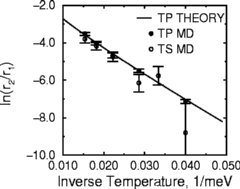 Joachim Jacobsen, Karsten Jacobsen (his former thesis advisor, but no relation:
the Danes have few names and they share them around), and I thought this was an
interesting question. If the rate for single jumps is determined by the height
of the barrier between wells, what determines the rate for double jumps?
Joachim and Karsten first ran some simulations, with realistic ``effective
medium'' simulations for Platinum, and found that the computer agreed that
double-jumps would happen a fair fraction of the time (although not as big
a fraction as the experiments: we don't have perfect simulations yet!)
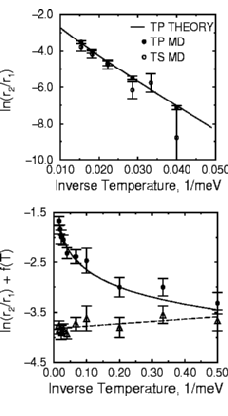
Their data is shown in the figure at right. Notice that we plot the
log of the fraction r2/r1, as a function
of one over the temperature: this way, a straight line means the
Arrhenius form is a good fit.
Joachim Jacobsen, Karsten Jacobsen (his former thesis advisor, but no relation:
the Danes have few names and they share them around), and I thought this was an
interesting question. If the rate for single jumps is determined by the height
of the barrier between wells, what determines the rate for double jumps?
Joachim and Karsten first ran some simulations, with realistic ``effective
medium'' simulations for Platinum, and found that the computer agreed that
double-jumps would happen a fair fraction of the time (although not as big
a fraction as the experiments: we don't have perfect simulations yet!)
Their data is shown in the figure at right. Notice that we plot the
log of the fraction r2/r1, as a function
of one over the temperature: this way, a straight line means the
Arrhenius form is a good fit.
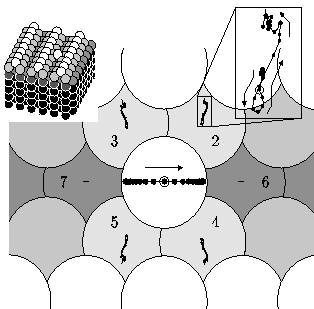 We then searched for the lowest energy path that would take an atom from the
top of one barrier to the top of the other, to get the energy
E2 needed for a double jump.
(We used brute force. We first
found a bunch of paths which made it, and averaged their positions and
velocities as they crossed the bottom of the well. We then minimized the
bejesus out of it.) You can see in the figure on the left what the path
ends up looking like: the motions of all the atoms but the central
one have been magnified by a factor of three.
The central atom starts at rest at the top of one well. It rolls down,
and rolls up the next well.
Partway down, it hits atoms two and four, and slows down. Normally
it would wiggle a bit more and then stop, forming a single jump. Instead,
atoms three and five kick it in the rear, propelling it over the top.
At late times, it creeps up to the top of the second hill.
We then searched for the lowest energy path that would take an atom from the
top of one barrier to the top of the other, to get the energy
E2 needed for a double jump.
(We used brute force. We first
found a bunch of paths which made it, and averaged their positions and
velocities as they crossed the bottom of the well. We then minimized the
bejesus out of it.) You can see in the figure on the left what the path
ends up looking like: the motions of all the atoms but the central
one have been magnified by a factor of three.
The central atom starts at rest at the top of one well. It rolls down,
and rolls up the next well.
Partway down, it hits atoms two and four, and slows down. Normally
it would wiggle a bit more and then stop, forming a single jump. Instead,
atoms three and five kick it in the rear, propelling it over the top.
At late times, it creeps up to the top of the second hill.
How weird! We thought that the atom would start with some zip, in order to have enough energy to get up the other side. Instead, it starts at rest, and the extra energy comes from atoms three and five. But they start at rest too! We thought more about it, and figured out that the extra energy is coming from infinity. (That's what the waves are for in the triple-well picture above.) Waves of energy focus in from infinity, give the atom a perfect push up the side, and then radiate away again: this is the extra energy E2-E1 needed for a double jump.
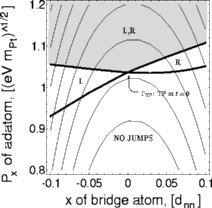 To finish the calculation of the rate
r2/r1, we needed to look at how many
of the paths near the optimal path would get over the barrier.
We were surprised to find that the region of double-jump paths (grey in the
figure at right) had a cusp at the perfect path. The figure shows
two of the many coordinates in phase space: the two black lines are the
curves dividing successful and unsuccessful crossings of the right and
left barrier. The optimum double-jump path we found is borderline for
both right and left, and the double-jump region has a kink right there!
To finish the calculation of the rate
r2/r1, we needed to look at how many
of the paths near the optimal path would get over the barrier.
We were surprised to find that the region of double-jump paths (grey in the
figure at right) had a cusp at the perfect path. The figure shows
two of the many coordinates in phase space: the two black lines are the
curves dividing successful and unsuccessful crossings of the right and
left barrier. The optimum double-jump path we found is borderline for
both right and left, and the double-jump region has a kink right there!
 This changes the equation for r2, multiplying
it by the square-root of the temperature T:
This changes the equation for r2, multiplying
it by the square-root of the temperature T:
How did we compare to the experiment? Since our computer atoms aren't quite the same potentials as the real atoms, we don't expect perfection. We got just about the right value for E2, but we didn't get as many double jumps as the experimentalists saw (they saw a few percent at a temperature where we saw around one percent).
Since then, our colleagues Montalenti and Ferrando in simulations of a similar gold surface found a different kind of path: the atom climbs up the side of the trough and jumps along the side one or more steps before it falls down again. For gold, this second kind of path contributes almost as many double jumps as our straight double jump, and it has a similar activation barrier (so it'll be competitive at lower temperatures too). Karsten tells us that similar paths are important for our platinum surface too, if we use a more accurate method for calculating the atomic potentials.
James P. Sethna, sethna@lassp.cornell.edu
![]() Statistical Mechanics: Entropy, Order Parameters, and Complexity,
now available at
Oxford University Press
(USA,
Europe).
Statistical Mechanics: Entropy, Order Parameters, and Complexity,
now available at
Oxford University Press
(USA,
Europe).