

|
 |
Amphiphiles as SurfactantsDefinitionsWe know that amphiphilic molecules possess a special molecular structure -- contain both a water loving polar part (hydrophilic) and a water hating non-polar part (hydrophobic). This property turns out to be very important in determining the special structures formed by amphiphilic molecules. The formation of these special structures is responsible for the use of amphiphiles in a large number of applications ranging from oil recovery to efficient delivery of drugs at a desired site in the body to their use in soaps and detergents. Amphiphiles are a special class of surface active molecules called surfactants . They are called surface active because they have the unique properties of getting adsorbed at various interfaces(e.g air-water, oil-water etc) and altering the properties of the interface. The hydrophilic part of an amphiphilic molecule (usually called the head) is a polar molecule whereas the hydrophobic part (called the tail) is a hydrocarbon chain 8-16 Carbon atoms in length.
Let us first try to understand how (or why?) special structures are
formed.
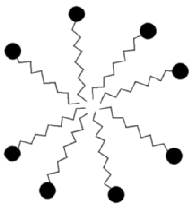
Depending upon the relative strength of these forces different structures tend to form . If the interaction between the polar heads is strong relative to the interaction between the non-polar parts than the molecules tend to form micelles (from the Latin 'micella' - meaning small bit) .
The stability of the micelles formed also depends upon whether the
concentration of molecules is greater than or less than a critical micelle concentration.
On changing the concentration of the amphiphilic molecules the resultant
structures formed changes. For example the lamellar phase forms at high
concentrations (this is also known as the smectic phase of lyotropic
liquid crystals.) .
The property of amphiphilic molecules that invariably plays a role in all applications is their ability to reduce the interfacial tension of the interfaces. A simple consequence of the property of lowering the interface tension
is their ability to act as emulsifiers - that is the phenomenon of otherwise
immiscible fluids becoming miscible.
Having understood how interfacial tension is reduced and that different
structures are formed at different concentrations let us explain
some applications of these structures. We will deal with
|
|
|