Most solids are made of crystals. A crystal is a regular, repeating arrangement of atoms. The simplest crystal conceptually is the so--called simple cubic structure, where the atoms lie on a grid: layers of rows and columns. Simple cubic crystals are rather rare, but many crystals form body-centered-cubic or face-centered-cubic structures, which can be viewed as simple cubic grids with an extra atom at the center of each cube or each face of a cube, respectively.
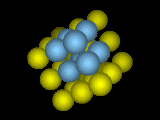 Simple Cubic Crystal.
Simple Cubic Crystal.The atoms form rows and columns and layers: they sit at the corners of stacked cubes.
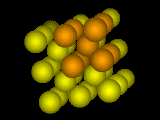 Body-Centered Cubic Crystal.
Body-Centered Cubic Crystal.One atom sits in the center of each cube.
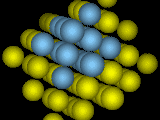 Face-Centered Cubic Crystal.
Face-Centered Cubic Crystal.One atom sits in each "face" of the cube. Notice the planes of atoms stacked "like cannonballs".
What crystal a given element will form is a subtle and complicated question. A few general guidelines are known. Simple cubic crystals with only one element are rare, mostly because they're floppy and tend to distort. Body--centered cubic (bcc) packings of atoms tend to be most stable near the melting point because the atoms have room to vibrate (important at high temperatures): face--centered cubic (fcc) crystals are common among metals at low temperatures because the atoms pack well. When you consider compounds or alloys made of more than one element, the variety of different crystal structures seen is quite bewildering.
![]() Jim Sethna, sethna@lassp.cornell.edu
Jim Sethna, sethna@lassp.cornell.edu
![]() Statistical Mechanics: Entropy, Order Parameters, and Complexity,
now available at
Oxford University Press
(USA,
Europe).
Statistical Mechanics: Entropy, Order Parameters, and Complexity,
now available at
Oxford University Press
(USA,
Europe).