When you go on a trip across the Atlantic, your body takes weeks to realize that it's not still at home. Even after a sleepless night, most of us will still have trouble falling asleep at the "right" time the next evening; you have an internal clock that refuses to reset until after several days of prodding. This jet-lag is the symptom of the circadian rhythms that keep a 24-hour clock in many of your cells, and indeed in most living things.
The study of circadian rhythms is fascinating. A complex network of interacting proteins sets up an oscillation once every 24 hours. A big mystery arises for organisms like bacteria and fish and reptiles that aren't warm blooded. Since the chemical reaction rates for proteins usually vary rapidly with temperature, how can the oscillation time overall be temperature independent? If all the individual rates precisely doubled, for example, it's easy to see that the fish would think a day had twelve hours.

|
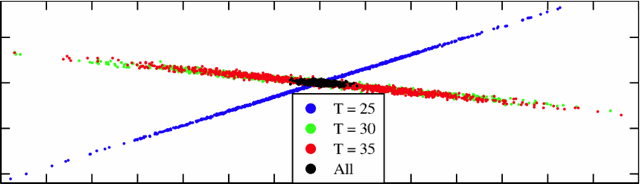
| How to keep your internal clocks on time. A two-dimensional view of the 36-dimensional parameter sets that have temperature-independent behaviors in the model of circadian rhythms. The colored dots are parameter sets that work only at one temperature; the black dots work at all temperatures (are robust to temperature variations). Notice that each temperature shows one stiff (thin) direction and a sloppy (long) direction. The other 34 dimensions "perpendicular" to this graph are also mostly sloppy. The acceptable region rotates and shifts with temperature, but the sloppiness allows different temperatures to intersect (robust black region) even though all rates are strongly temperature dependent. |
We think the answer has to do with the fact that circadian rhythms is a sloppy model. Sloppy models are multiparameter systems whose overall behaviors depend only on a few "stiff" combinations of parameters. The figure with the colored cross shows the parameters that work at different temperatures. A whole thin line of parameters works for each temperature, and so it's not such a surprise that there are rates that work at all temperatures. In fact, in the 36-dimensional space of parameters, the ones that work fill a wide, flat surface with large width along most of the directions: the intersection of these three surfaces are the ones that give temperature independent behavior.
More precisely, so long as the stiff direction depends on the environmental perturbation, the system can use sloppy modes to yield robust behavior. For example, the low-temperature blue parameter region are tightly constrained along a different stiff direction than the red and green parameter sets in the figure above. By sliding along its sloppy directions (anything perpendicular to a few stiff directions), the low temperature system can keep inside the blue region (fixed low-temperature behavior), and tune itself to also sit inside the red and green regions (robust to environmental changes).
|
|
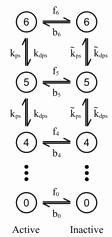
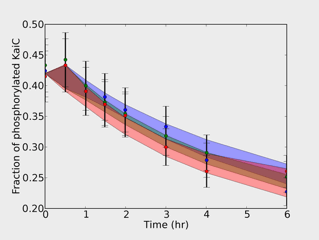
| Circadian KaiC Phosphorylation Network | Robust. Experimental slow time-decay of phosphorylation, along with the model predictions in the black "robust" parameter region; both are largely temperature independent. |
The model we use was developed by others for circadian rhythms in blue-green algae (cyanobacteria), which experimentalists have now duplicated in a test tube. The slow part of the reaction, it seems, is the addition and removal of up to six phosphate groups on one of the proteins, called KaiC, and with the switching of KaiC between two different shapes. The last two figures show the model, the experimental data (which is almost independent of temperature), and the range of data fits we get when we explore parameters that are robust to temperature changes (black dots above).
How can the decay (and the resulting circadian rhythm) take the same time, if all the reactions roughly double in speed from 25 to 35 degrees Centigrade? All the proteins start in the active state. The robust parameters, we find, mostly remove phosphate groups (dephosphorylation faster than phosphorylation) for the active proteins, and add phosphate groups for the inactive proteins. As the temperature rises, the rate that active proteins change into inactive proteins increases. If it increases faster (has a higher activation barrier) than the phosphorylation and dephosphorylation rates, then at higher temperatures the proteins will spend more time inactive (getting more phosphate groups), and thus compensate for the speedier removal of phosphate groups from those that remain active. All the rates can increase, but if some increase faster than others, the net rate can stay constant.
The bottom line, though, is that this isn't a delicate balancing act. Because
the system is sloppy, there are huge regions of parameters that work at
each temperature, and it's easy to arrange for parameters that work for
all temperatures (choosing inside the intersections of each). Robustness
to environmental changes (like temperature) is not difficult if the model
is sloppy...
James P. Sethna, sethna@lassp.cornell.edu; This work supported by the Division of Materials Research of the U.S. National Science Foundation, through grant DMR-070167.
![]() Statistical Mechanics: Entropy, Order Parameters, and Complexity,
now available at
Oxford University Press
(USA,
Europe).
Statistical Mechanics: Entropy, Order Parameters, and Complexity,
now available at
Oxford University Press
(USA,
Europe).